Abstract 1. Colloidalalloyswith preassembled clusters and spheres Self-assembly is a powerful method for constructing colloidal crystals, mainly because of spheres, rods...
Colloidal alloy
(Colloidalalloys with preassembled clusters and spheres)
Self-assembly is a powerful method for constructing colloidal crystals, mainly because spheres, rods or multi-faceted particles can build countless structures. However, many complex or low coordination structures, such as diamonds, pyrochlore, and other lattices, cannot be self-assembled. Ducrot et al. introduced a new pre-assembled component based on the desired superstructure and the design principles of programmed nearest DNA modulating interactions to create structures that would otherwise be impossible. Using pre-assembled colloidal tetrahedra and spheres, they obtained cubic and tetragonal colloidal crystals without known atomic analogs and osmotic low-coordinate diamond and pyrochlore sublattices that had never been assembled before. (Nature Materials DOI: 10.1038/NMAT4869)
2. Simulating the anti-fog ability of nano-texture
(Antifogging abilities of modelnanotextures)

Nanoscale features with special shapes give the insect surface a wide range of unique properties. These properties are essential for the survival of animals, such as the low light reflectance of moth eyes, the oil repellency of the carapace shells and the superstickiness of palm worms. Anti-reflective mosquito eyes and scorpion fins are also known to have some anti-fog and self-cleaning properties. In all of these cases, the combination of small feature sizes and optimal shape provides excellent surface properties. Mouterde et al. studied the anti-fog mechanism using model materials that mimic natural systems and pointed out the importance of the size and shape of the texture. Although exposure to mist greatly detracts from the hydrophobicity of the hydrophobic structure, this failure can be minimized by scaling the structure to nanometer size. If the hydrophobic surface consists of nano-cones, it will produce an atomization efficiency close to 1, and water droplets less than 2 μm will detach, and the original impairment effect can even become too small to be measured. (NatureMaterials DOI: 10.1038/NMAT4868)
3. Ultra-hardness and toughness hydrogel
(Enzymatic mineralization generateultrastiff and tough hydrogels with tunable mechanics)
Animal cartilage and skin are composed of more than 50% water, but are quite hard (with a modulus of elasticity of up to 100 MPa), tough and difficult to break (breaking energy up to 9000 joules per square meter). These features make these biomaterials physically superior to existing synthetic hydrogels. Recently, much progress has been made in the synthesis of tough hydrogels, which achieve skin-like toughness and inorganic-organic composites exhibit better performance.
However, these materials have high tensile properties due to their toughness; synthetic hydrogels cannot compete with their natural counterparts in terms of stiffness, and the best examples have only 10 MPa or less elastic modulus. the amount. Rauner et al. have described enzyme-induced precipitation and crystallization of calcium carbonate containing hydrogels, but the resulting material is brittle. This time they reported that the enzyme induced the formation of amorphous calcium phosphate nanostructures, which were evenly distributed in the polymer hydrogel. The best of these materials have a breaking energy of 1300 Joules per square meter even in their fully water-swellable state, which is superior to most known water-swellable synthetic materials. They are also able to adjust their stiffness up to 440 MPa, far more than cartilage and skin. (Nature DOI: 10.1038/nature21392)
4. Electrolyte additive to achieve rapid charging and stable cycle of lithium metal battery
(Electrolyte additiveenabled fast charging and stable cycling lithium metal batteries)

A battery using a lithium (Li) metal as an anode is considered to be a promising energy storage system because of its high energy density. However, the safety issues associated with dendrite growth and limited cycle life (especially at high charge current densities) hamper their practical application. Zheng et al. adjusted the LiPF6 additive to the optimum amount (0.05M) in the LiTFSI-LiBOB double salt/carbonate solvent-based electrolyte, which significantly enhanced the charging ability and cycle stability of the Li metal battery. This optimum addition amount allows the Li metal battery of the 4V Li ion cathode to have a capacity retention rate of 97.1% after 500 cycles of a moderately high load of 1.75 mAh·cm-2, and a charge current density of 1.75 mA. • The increase in the overpotential of the lower electrode of cm-2 is very limited. The fast charge and stable cycle performance is attributed to the stable and electrically conductive solid electrolyte interface created on the Li metal surface as well as the stable Al cathode current collector. (Nature Energy DOI: 10.1038/nenergy.2017.12)
5. Evidence for anion redox activity in three-dimensional ordered Li-rich positive electrode β-Li2IrO3
(Evidence foranionic redox activity in a tridimensional-ordered Li-rich positive electrodeβ-Li2IrO3)
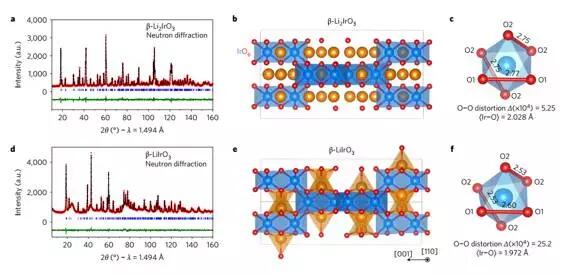
The choice of cathode materials for lithium ion batteries has been dependent on cationic redox reactions until the redox activity of anions in Li-rich layered compounds was recently discovered, resulting in capacities up to 300 mAh·g-1. There has been an unresolved problem in the search for high-capacity electrodes for anion redox, which is about the importance of the structural dimension.
Pearce et al. reported a β-Li2IrO3 phase, although Ir arranged in a three-dimensional (3D) framework is not like the typical two-dimensional (2D) layer seen in other Li-rich oxides, but each lr can be reversibly Exchange 2.5e-, the highest value in any intervening reactions involving d-metals reported. Such a large degree of activity comes from the combination of a reversible cation (Mn+) and an anion (O2)n- redox process, which can be observed by transmission electron microscopy and neutron diffraction experiments, and confirmed by theoretical calculation of density function. In addition, β-Li2IrO3 exhibits good cycle characteristics while not exhibiting the cation migration and shear atomic layer seen in the 2D layered Li-rich material. It is worth noting that the anionic redox process, together with Ir4+ oxidation, occurs at a potential as low as 3.4 V relative to Li+/LiO, which is the same as observed in the layered α-Li2IrO3 polycrystal. Theoretical calculations illustrate the electrochemical similarities and differences in the structural, electronic, and mechanical descriptions of 3D and 2D polycrystals. The discovery by Pearce et al. frees structural size constraints and offers more possibilities for designing high energy density electrodes for next-generation lithium-ion batteries. (Nature Materials DOI: 10.1038/NMAT4864)
6. Low band gap mixed tin-lead iodide perovskite absorbent
(Low-bandgap mixedtin–lead iodide perovskite absorbers with long carrier lifetimes forall-perovskite tandem solar cells)
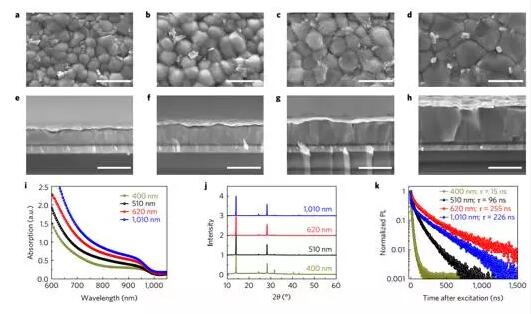
Tandem solar cells using only metal halide perovskite sub-cells are one of the attractive options for next-generation solar cells. However, the lack of high performance low bandgap perovskite solar cells has hampered the development of highly efficient perovskite tandem solar cells. Zhao et al. reported a highly efficient mixed tin-lead iodide low bandgap (~1.25 eV) perovskite solar cell with an open circuit voltage of up to 0.85 V and 700-900 nm in the infrared wavelength range of over 70%. A short-circuit current density exceeds 29 mA·cm-2 and is suitable for the underlying battery in a perovskite tandem solar cell. The low band gap perovskite solar cell achieves a maximum power conversion efficiency of 17.6% and an empirical efficiency of 17.01%, and the current-voltage hysteresis is negligible. This best perovskite 4-terminal tandem solar cell exhibited a steady state efficiency of 21.0% when mechanically stacked with a perovskite top cell with a band gap of about 1.58 eV. (Nature Energy DOI: 10.1038/nenergy.2017.18)
7. Covalent organic framework compounds
(The atom, the molecule, and the covalentorganic framework)
About a century ago, Lewis published a groundbreaking work on covalent bonds, and since then covalent bonds have been playing an important role in the theory of making organic molecules. With the advent of covalent organic framework compounds (COFs), covalent bond chemistry has expanded to two-dimensional three-dimensional frameworks. Yaghi et al. reviewed the development of porous light-element (B, C, N, O and Si) COFs synthesized by covalent bonding of organic molecules. The discovery of these COFs laid the foundation for the framework compounds to lose porosity and crystallinity and to achieve the desired material properties. (ScienceDOI: 10.1126/science.aal1585)
8. High ORR active sub-nano Pt alloy nanowires
(Efficient oxygenreduction catalysis by subnanometer Pt alloy nanowires)
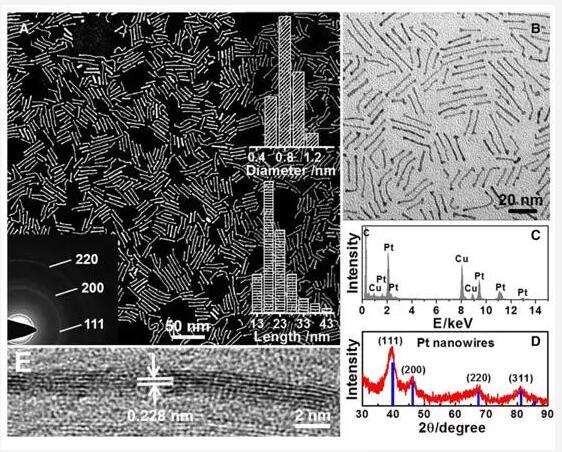
Pt and Pt alloy nanoparticles (NPs) below 2 nm are generally considered to be unsuitable for oxygen reduction reactions (ORR). However, whether the same trend applies to Pt-based nanowires (NWs) and nanosheets remains to be solved because there is no scalable method for preparing these Pt nanostructures. Jiang et al. reported a general method for preparing Pt alloy NWs with a diameter of only 4-5 atoms, including single metal PtNWs, bimetallic PtNi, PtCo NWs, and trimetallic PtNiCo NWs. In stark contrast to Pt alloy NPs, sub-nanometer Pt alloy NWs exhibit exceptional mass and specific activity (0.2A vs. 4.2A/mg and 5.11 mA/cm2 at RHE), which is 32.3 higher than commercial Pt/C, respectively. And 26.9 times. Density functional theory simulation attributed this high ORR activity to the high density (111) crystal plane active sites on the sub-nanometer Pt alloy NWs. These catalysts also showed good stability with little attenuation after 30,000 cycles. (Science Advance DOI: 10.1126/sciadv.1601705)
Kylin Chemicals manufactures and markets high quality chelants and sequestrants, including EDTA, Tetrasodium EDTA,EDTA Disodium salts, EDTA Tetrasodium salt, HEDP, PBTC, ATMP and DETPMP, etc.

Our sequestrants are widely used in variety of applications like detergents & cleaners, cosmetics, industrial water treatment, oilfield water treatment, textile dyeing, etc.

EDTA Chelants & Phosphonate Sequestrants
Edta Chelation,Phosphonate Sequestrants,Ethylenediaminetetraacetic Acid,Phosphino Polycarboxylic Acid
Kylin Chemicals Co., Ltd. , http://www.kylin-chemicals.com