Peking University researches and develops modified garnet electrolytes
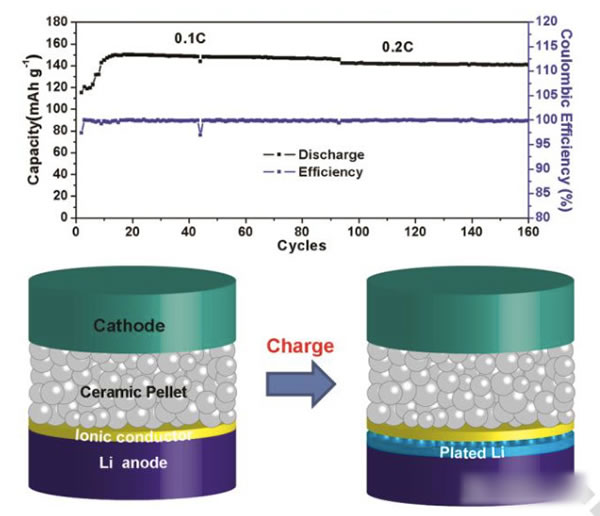
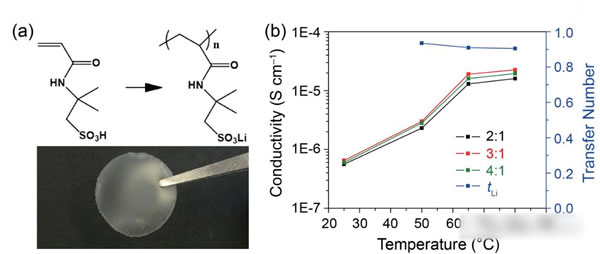
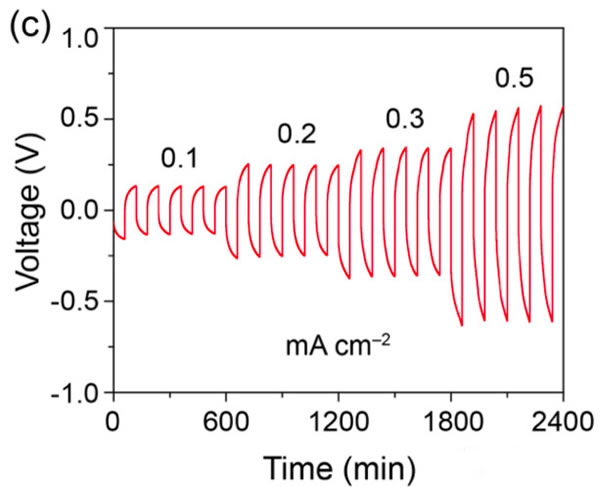
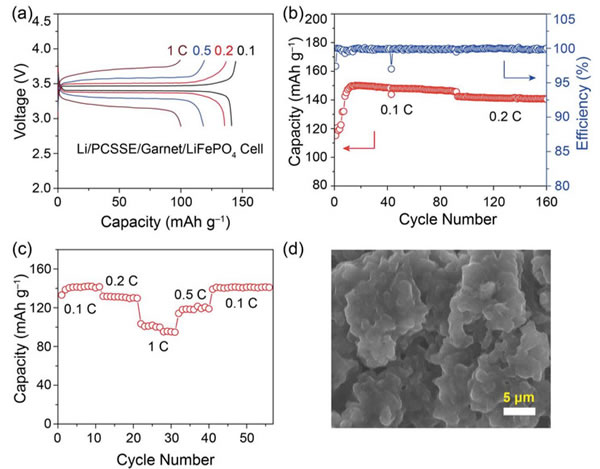
The specific energy of the power battery continues to increase. The existing high nickel material + silicon carbon system can achieve a maximum specific energy of about 350Wh / kg. To continue to increase the specific energy, a new system is needed. Solid-state metal Li battery, Li / sulfur battery and Li / air battery, from the current technical level, all-solid-state metal Li battery is the most likely next-generation high specific energy battery. For all solid-state batteries, solid electrolyte is the key technology. Generally speaking, the solid electrolyte has a low Li + conductivity at normal temperature, which affects the performance of the battery. In order to improve the conductivity of all-solid electrolytes, people have developed various types of all-solid electrolytes, of which the conductivity of garnet-type all-solid electrolytes at room temperature can reach 10-4-10-3S / cm, and common carbonates The liquid-like electrolyte 10-2S / cm is very close, it is an ideal all-solid electrolyte, but the garnet electrolyte also faces the surface inert layer (LiOH, Li2CO3) and metal Li has poor wettability, metal Li dendrite in the crystal Boundary growth, large interface impedance and other issues. Recently, Weidong Zhou (first author and corresponding author) of Peking University, Yutao Li (corresponding author) and John B Goodenough (corresponding author) of the University of Texas at Austin achieved this by coating a layer of Li + on the surface of the garnet electrolyte. The polymer electrolyte method of 0.9 suppresses the growth of metal Li dendrites and reduces the interface impedance, which makes the first Coulomb efficiency of the all-solid metal battery increase to 97%, and the Coulomb efficiency in the cycle is close to 100%. Garnet solid electrolytes have the problems of non-wetting interface, Li dendrite growth and poor interface contact, and the good mechanical properties of polymer electrolytes make them an effective way to solve this problem. The usual polymer electrolytes do not contain Li salts Therefore, it is necessary to add lithium salts such as LiTFSI, but this also makes the migration number of Li + tend to be relatively low (for example, 0.35), so the anion will gather on the side near the positive electrode during the charging process, thus generating a strong electric field, affecting Li + Diffusion accelerates the growth of Li dendrites in the garnet electrolyte, and the polymer electrolyte with a high Li + migration number has fewer mobile anions, which can effectively solve this problem and improve the performance of the garnet solid electrolyte. The structure of the polymer electrolyte used in the experiment is shown in the following figure (poly (acrylamide-2-methyl-1-propane sulfonate sodium) lithium PAS). In this molecular structure, only Li can move, so the number of Li + migration It is also very high, reaching around 0.9. In order to further improve the mechanical properties of PAS and Li + conductivity, the authors mixed PAS with PEO (polyoxyethylene). The interaction between PEO and PAS also promoted the movement of Li + along the long chain of PEO, as can be seen from the figure below To PEO: PAS = 3: 1, the conductivity of the electrolyte is the highest, it can reach 1.8x10-5S / cm at 65 ℃, because of the single ion conductivity, the migration number of Li + is as high as 0.87-0.95, indicating that most of the conductivity All contributed by Li +, which has a positive effect on reducing polarization, improving magnification and cycle performance. The conductivity of the garnet solid electrolyte can reach 4x10-4S / cm at room temperature and 1x10-3S / cm at 65 ℃, but its poor contact with the metal Li anode leads to an increase in impedance, so Weidong Zhou The stone electrolyte (450um thick) is combined with a layer of PEO-PAS polymer electrolyte (about 5um thick). Because the thickness of PEO-PAS is thin, although its conductivity is low, the impedance caused is relatively small. The composite electrolyte The overall Li + conductivity can reach 1.5x10-4S / cm. At the same time, due to the good mechanical properties of PEO-PAS, the contact resistance between the metal Li anode and the solid electrolyte is greatly reduced. In the absence of a polyelectrolyte coating, the interface impedance of Li / LLZTO / Li reaches 5000 ohms, while in garnet After adding a polymer coating to the electrolyte surface, the interface impedance dropped to 400 ohms. Another problem faced by the garnet solid electrolyte is the problem of Li dendrites growing along the grain boundary during the cycle. The ordinary garnet solid electrolyte is circulated in Li / LLZTO / Li battery for 5 hours. Obvious short circuit has occurred, and the garnet solid electrolyte after surface treatment with polymer electrolyte shows very stable cycling performance (as shown in the figure below, the current density is 0.1, 0.2, 0.3 and 0.5mA / g respectively The next cycle is 10 hours (charging 1h, then discharging 1h)), the electrolyte can circulate stably for more than 500 hours without short circuit. The figure below shows the electrochemical test results of a full battery made with Li / garnet electrolyte / LFP. As can be seen from the figure a below, the full battery shows good rate performance, and the positive electrode capacity can reach 145mAh / at 0.1C rate. g, the reversible capacity of the positive electrode reaches 140mAh / g at 0.2C rate. Of course, due to the large impedance of the all-solid battery, there is still a certain gap compared to the liquid electrolyte battery. It can be seen from Figure b that the battery's first coulombic efficiency can reach 97%, and after 160 cycles at 0.2C rate, the reversible capacity is still as high as 137mAh / g, and the battery's coulombic efficiency in the cycle reaches 99.9%, indicating that the battery Good electrochemical stability. After the battery was cycled, the author disassembled it, and the surface of the metal Li anode was deposited more uniformly, and there was no obvious trace of Li dendrite growth (as shown in figure d below). The conductivity of the garnet electrolyte is as high as 10-3S / cm. After modification of the surface with the PAS-PEO polymer electrolyte, Weidong Zhou effectively reduced the charge exchange resistance of the interface and promoted the uniform distribution of current , Thereby significantly inhibiting the growth of Li dendrites along the grain boundaries, avoiding the occurrence of short circuits, and greatly improving the cycle stability of the full battery. This technology has significantly improved the practicality of garnet electrolytes and is of great significance for the development of all-solid electrolytes. Bs 5896 PC Wire,Steel Strand Wire,PC Strand Prestressed Wire,Prestressed PC Steel Strand Shandong Xindadi Holding Group Co., Ltd , https://www.xindadipcwire.com